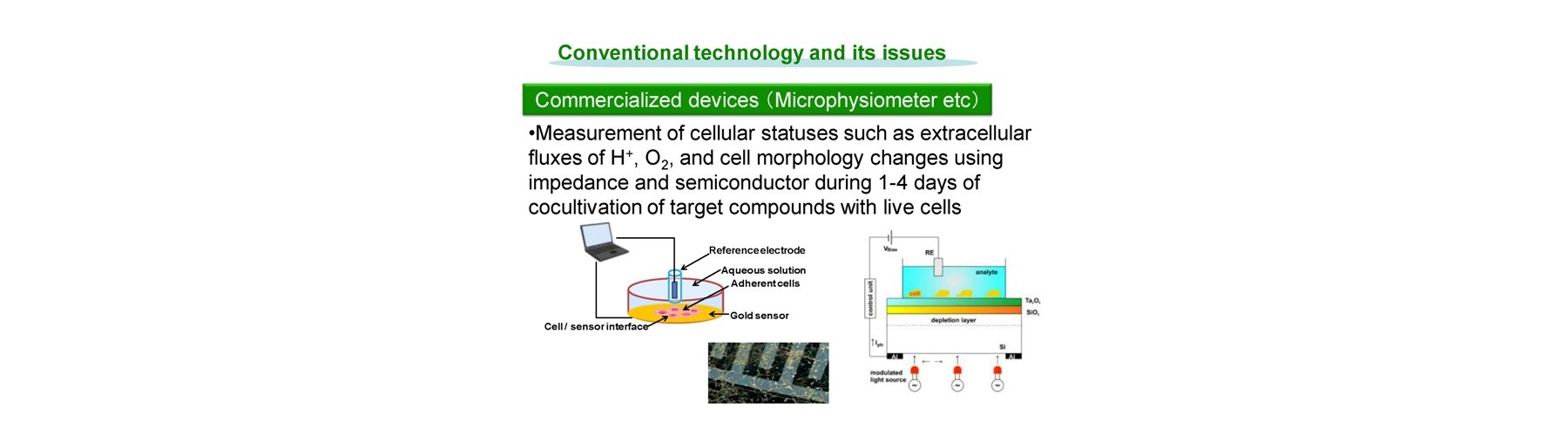
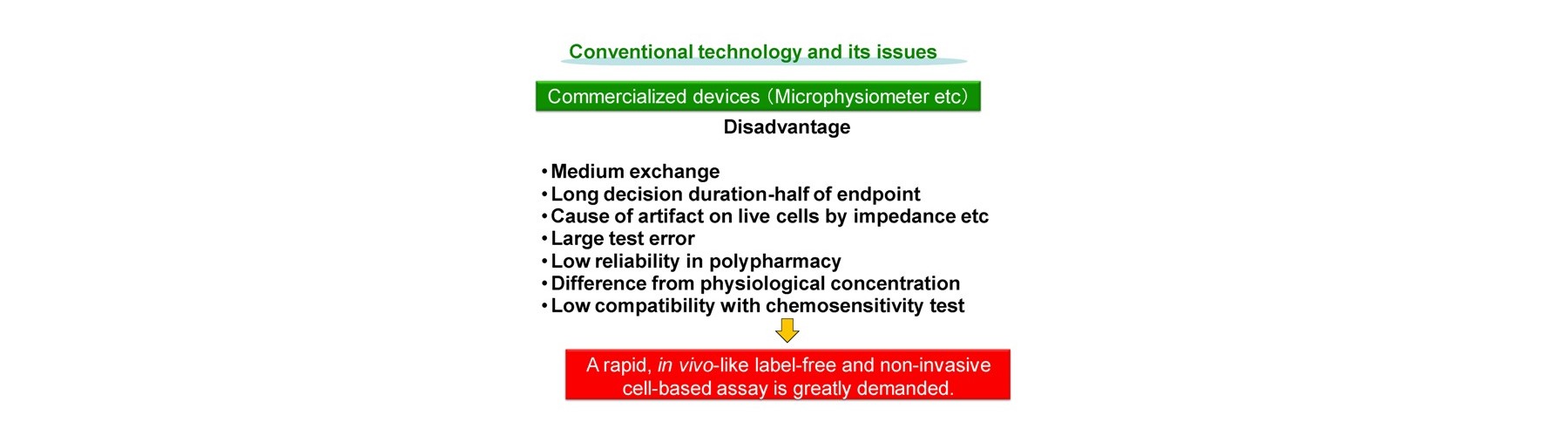
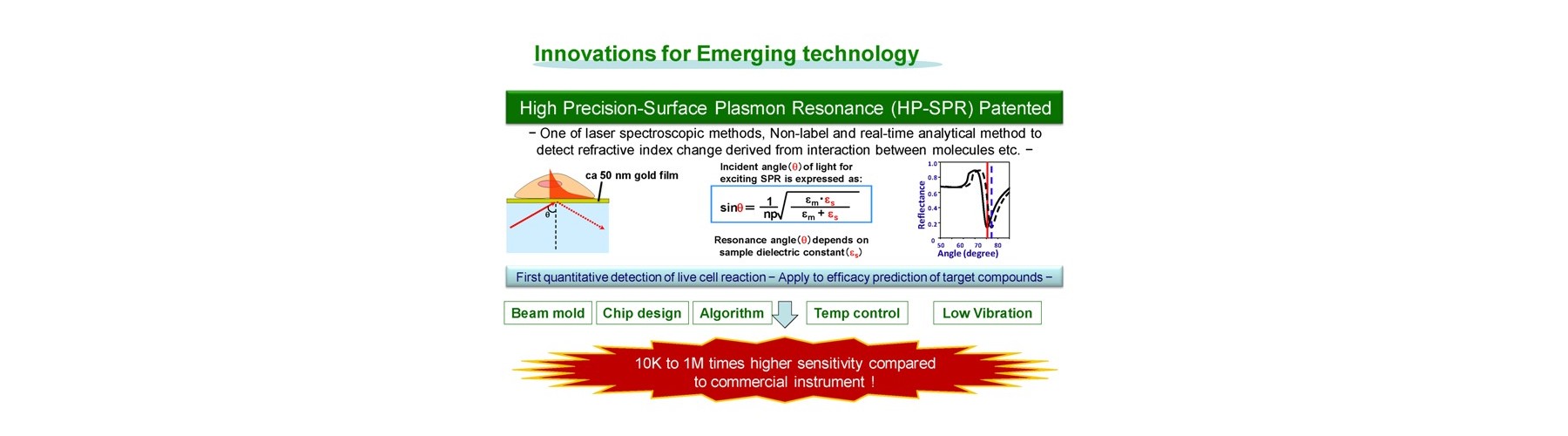
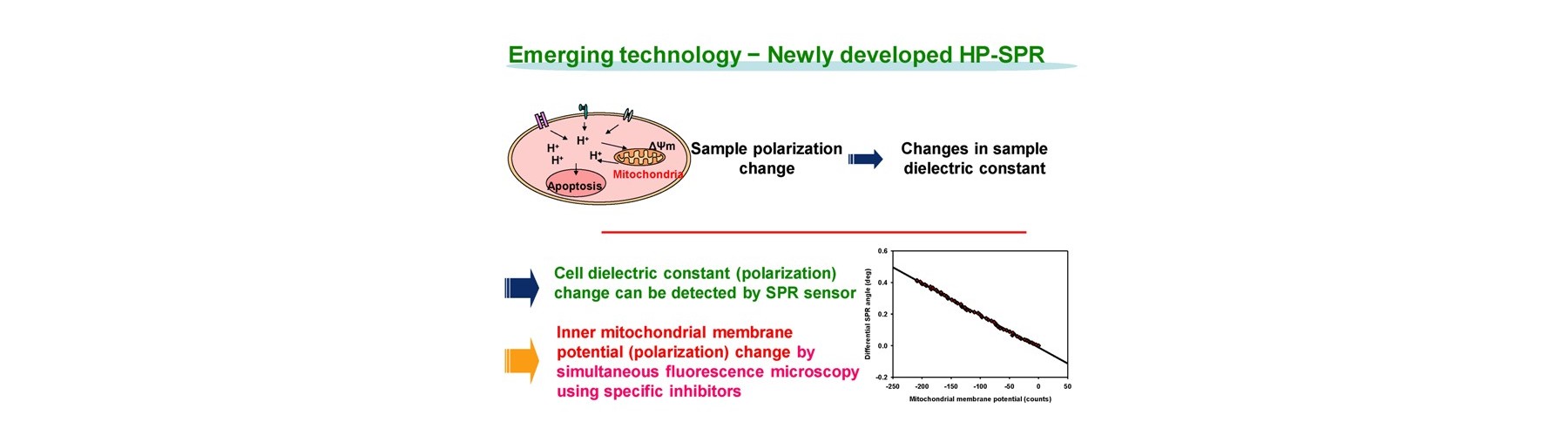
Chemosensitivity test
1. 1h in vivo-like phenotypic screening service on commission for medicine, food, functional food, cosmetics and their candidates
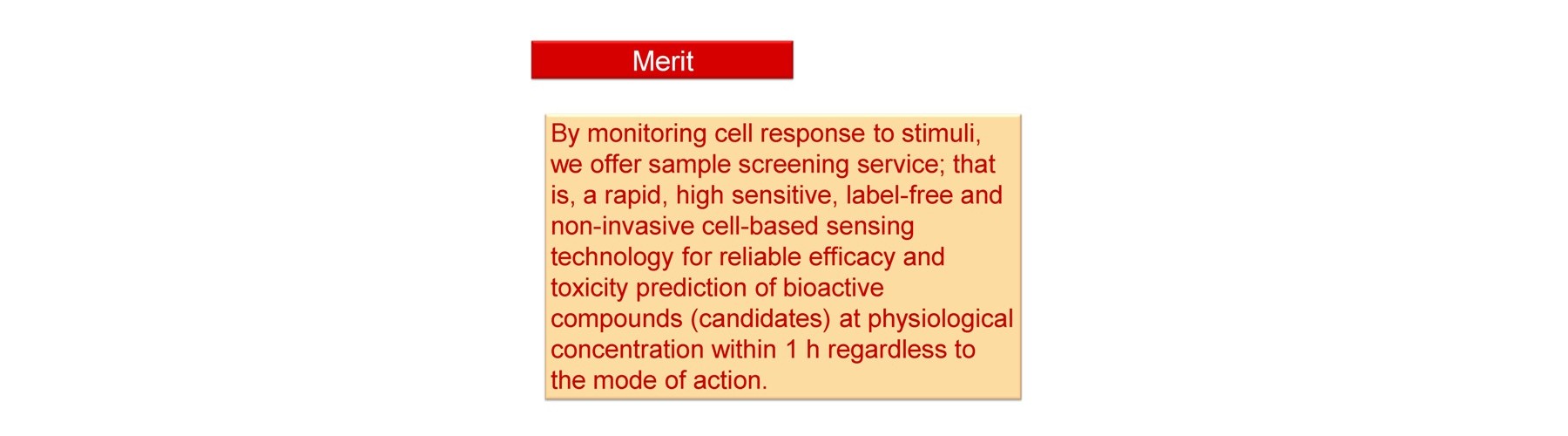
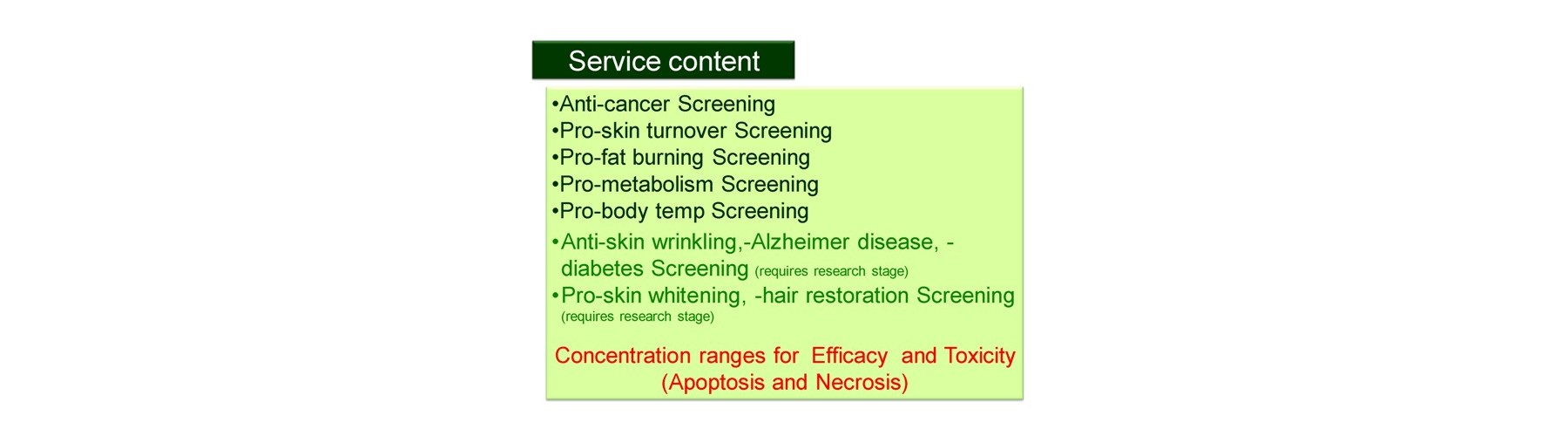
We provide in vivo-like test (screening) services against the following targets using human live cells.
After receiving your samples, we will feed back the conc. ranges for efficacy, toxicity expressed as apoptosis and toxicity expressed as necrosis.
-
- Anti-cancer
- Pro-fat burning
- Pro-metabolism
- Anti-aging
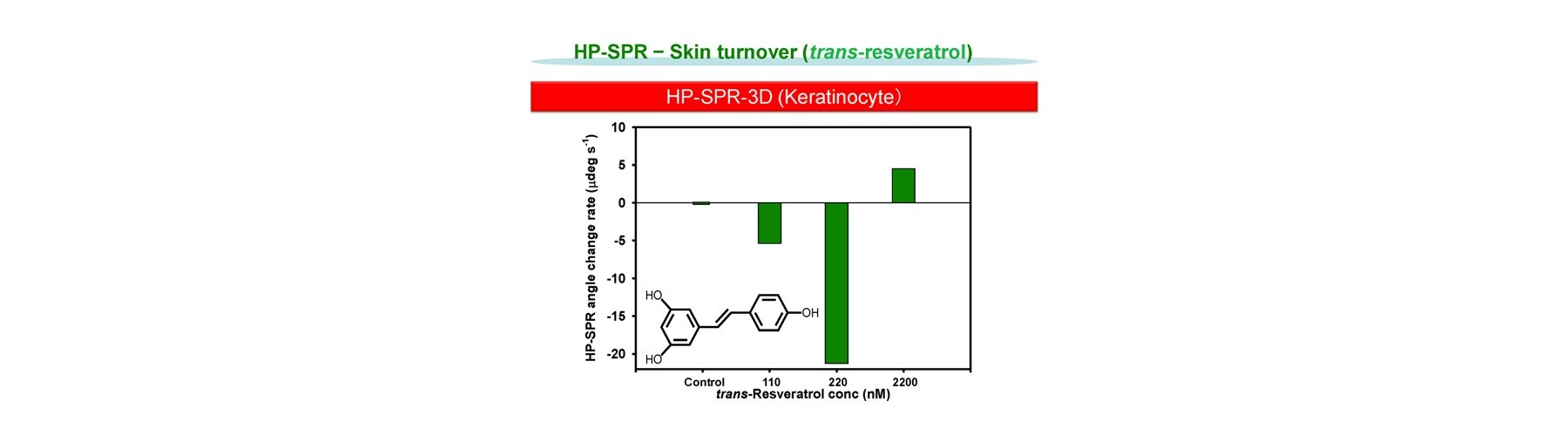
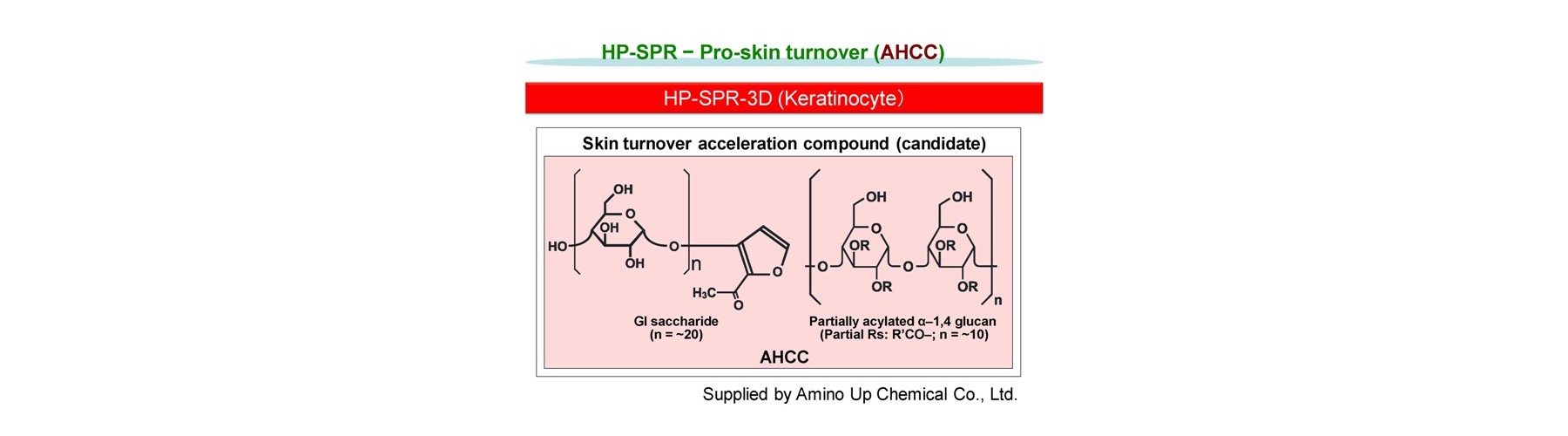
- Pro-skin turn over
- Pro-body temp
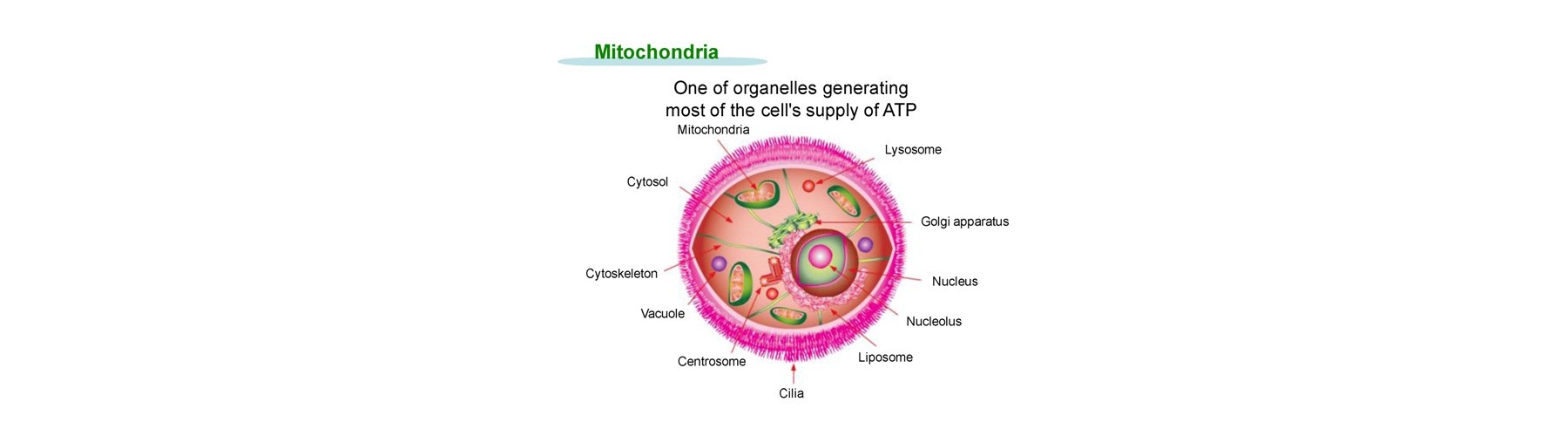
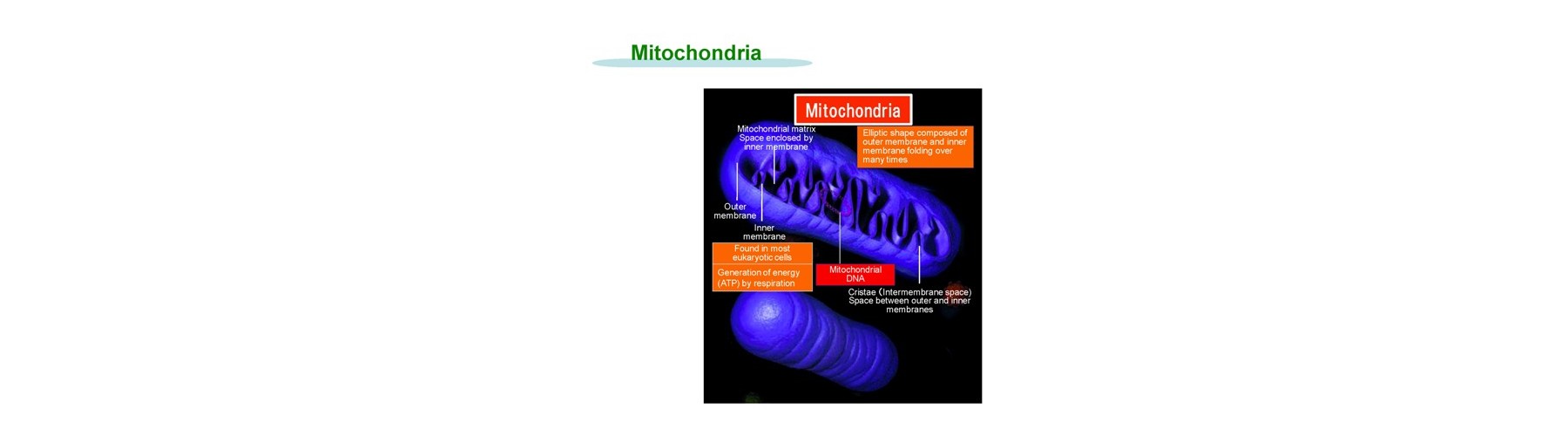
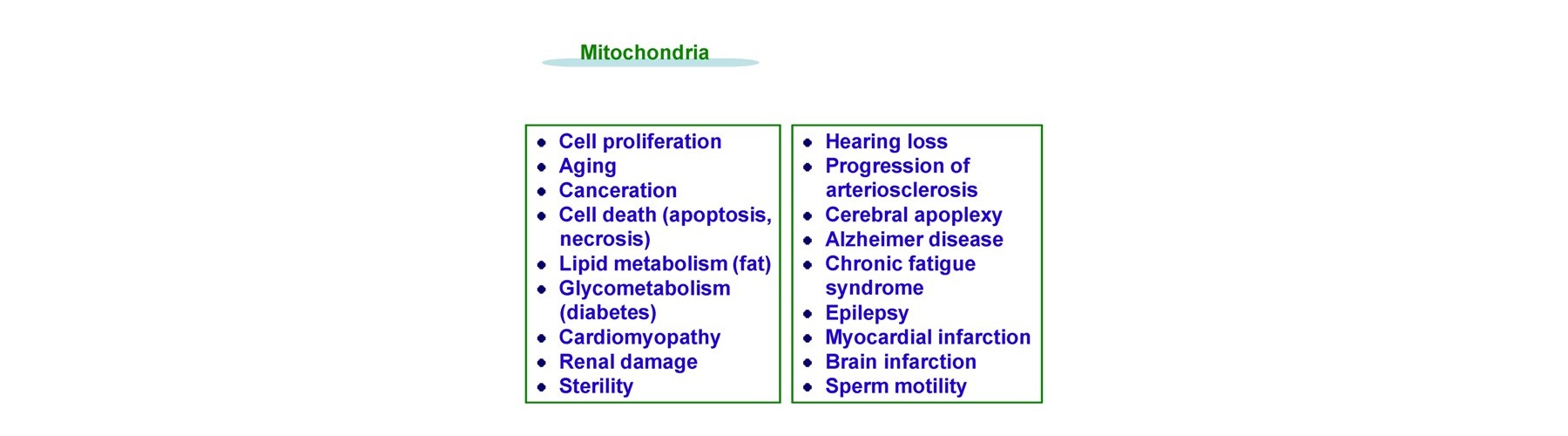
The expected applications relate to mitochondria and its examples are as follows.
- Anti-Alzheimer disease
- Anti –diabetes
- Anti-epileptic
- Pro -hair restoration
- Pro-skin whitening
- Anti-skin wrinkling
Our service is suitable for drug repositioning, and searches synergistic effect and rescue effect for combination use of compounds. This enables regeneration of old or discarded drugs to be patented. Furthermore, MitoPOM well determines drug efficacy and toxicity in case of drug delivery. Moreover, MitoPOM requires no cell culture and is very effective for new drug development especially in using stem cell disease model necessitating long term cell culture causing difficulty to keep the healthy cell condition.
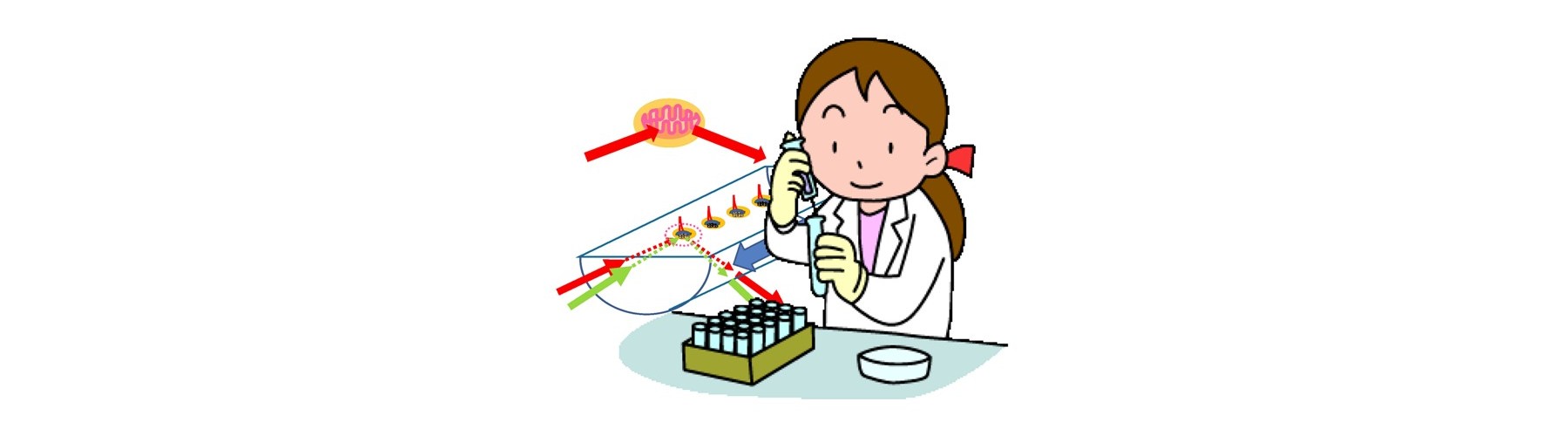
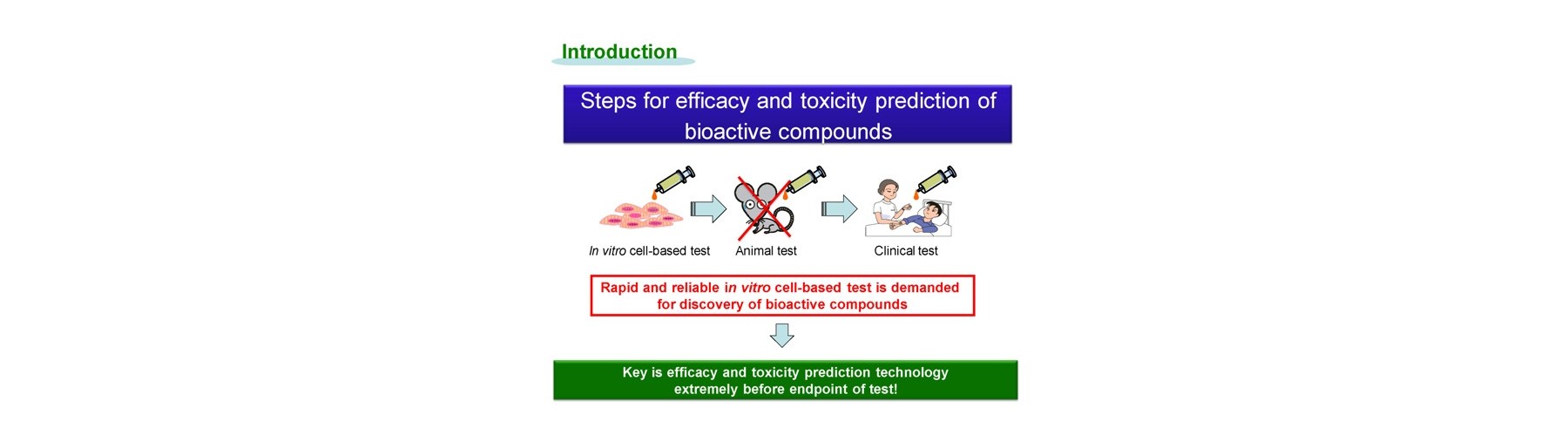
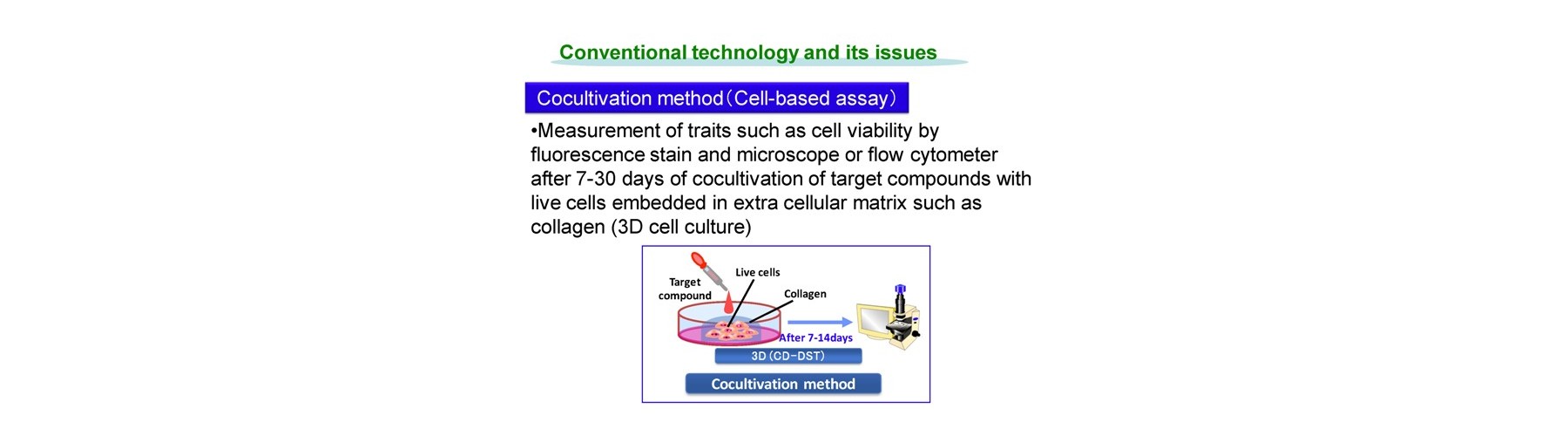
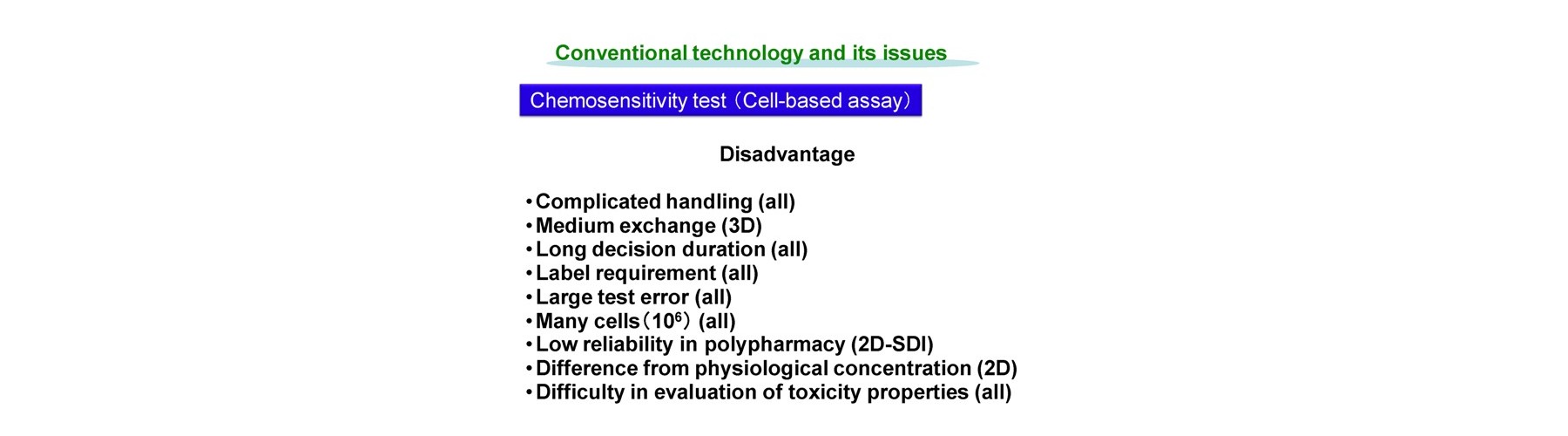
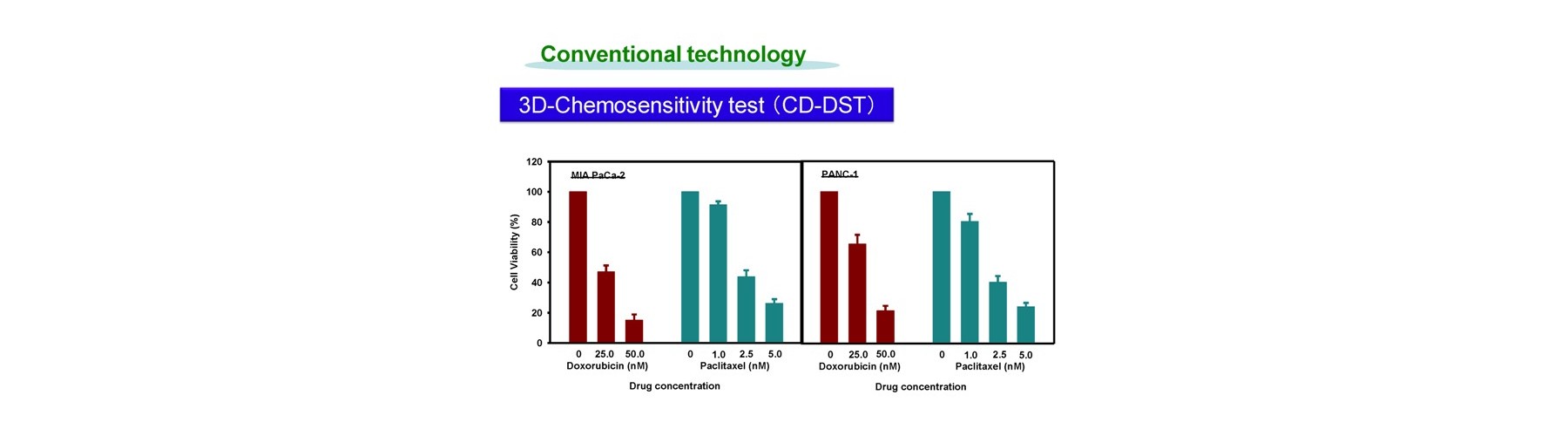
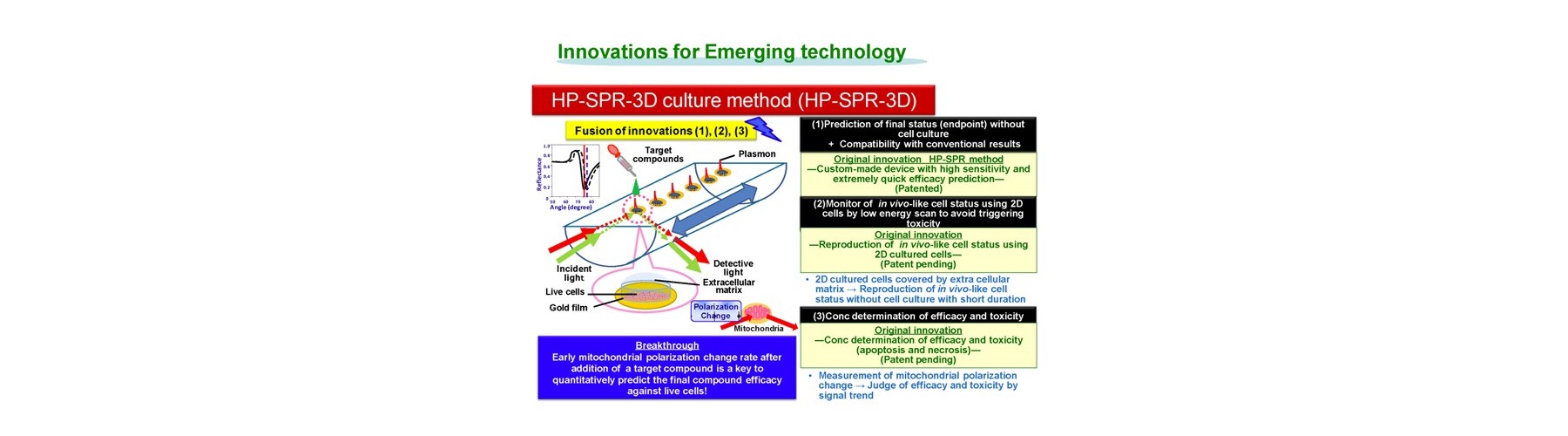
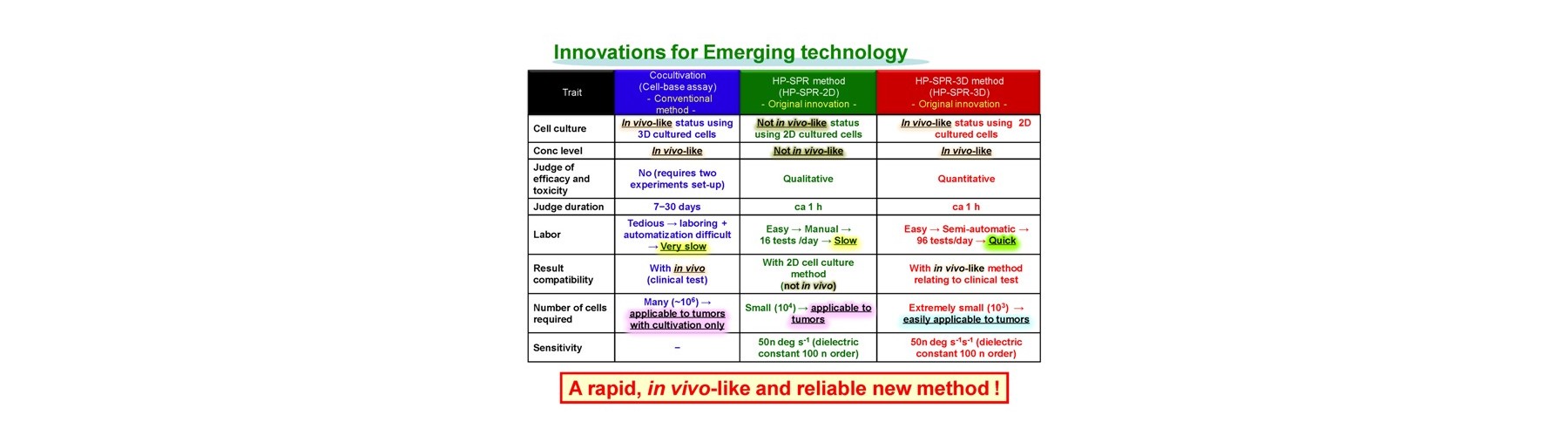
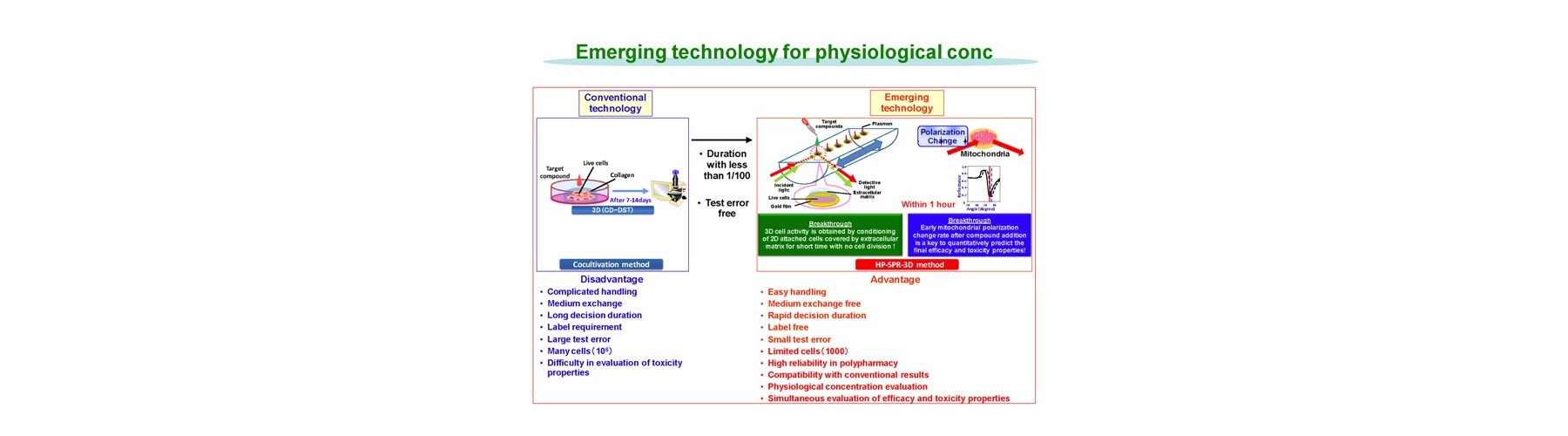
Conventional technology of in vivo-like test is based on expensive endpoint assay of 3D cell culture with extracellular matrix necessitating time-consuming experimental process (2-4 weeks) and labeling agents causing interference with a target compound that provide low reliability. The previous device methods depend on the pharmaceutical mode of action, and give no relation to the conventional method. And a separate set of experiment is required to obtain both efficacy and toxicity results. Our new technology overcomes these all problems sensing dynamic cellular reaction against target compound(s) by laser.
The technological break points are as follows.
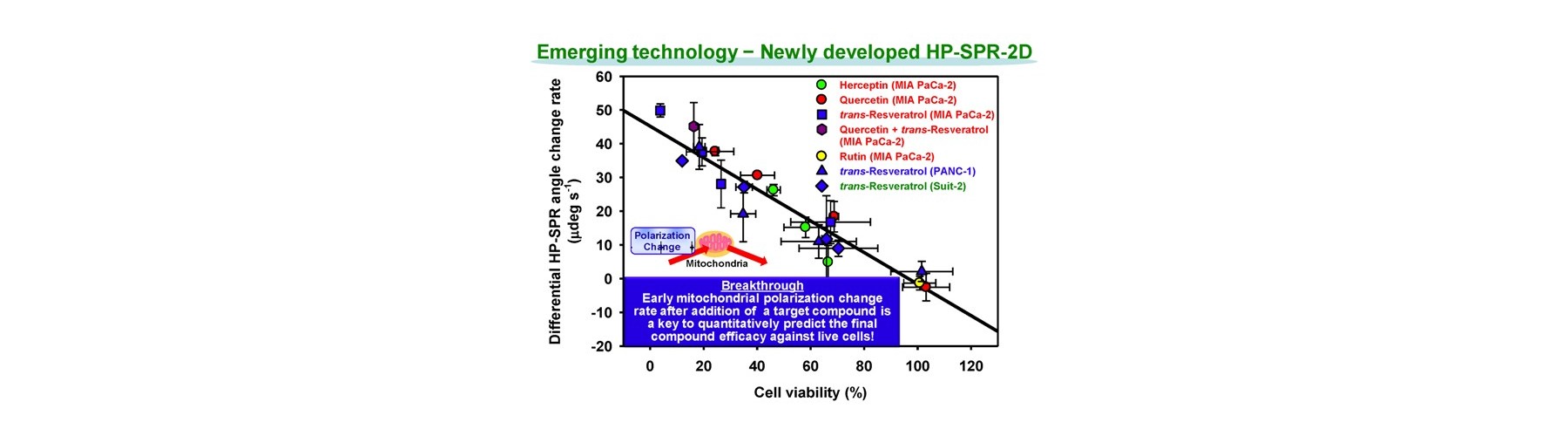
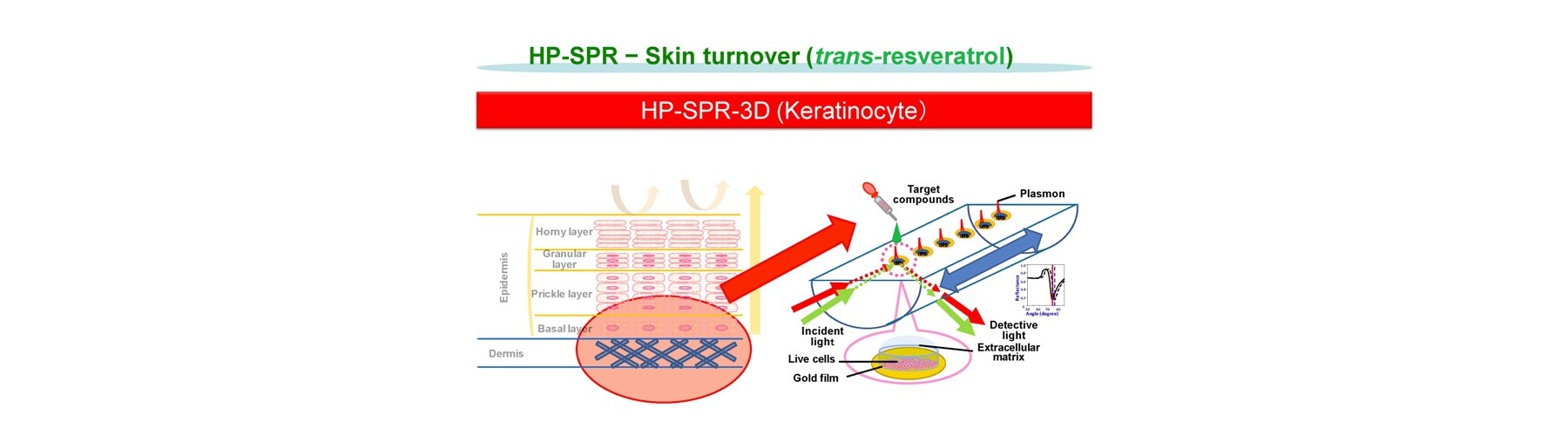
- New device is developed for real-time monitoring of mitochondrial polarization status within live cells without both labeling and invasion.
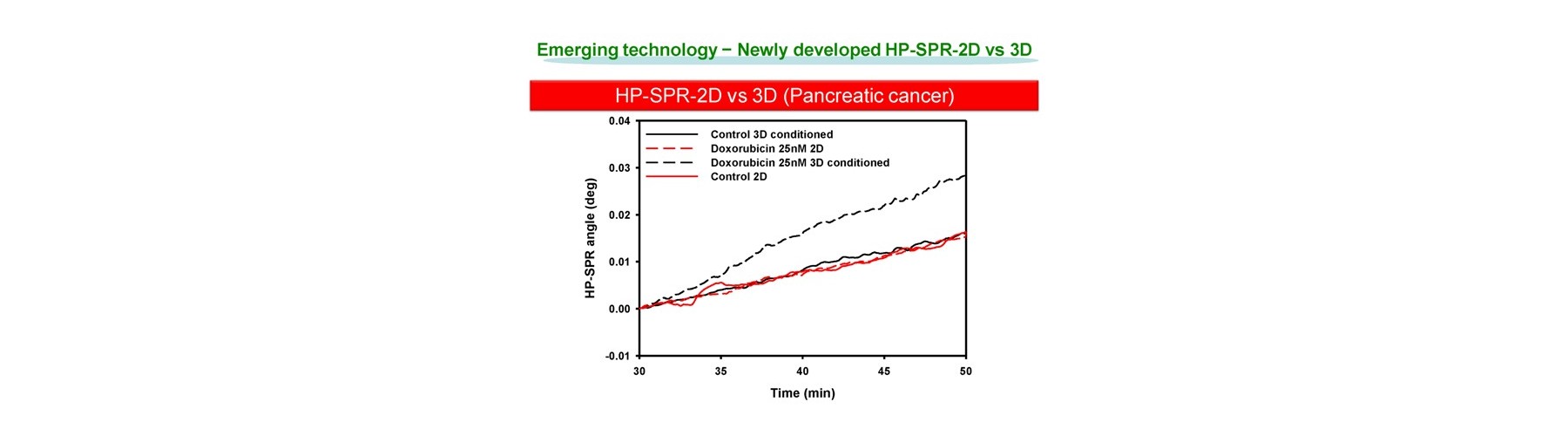
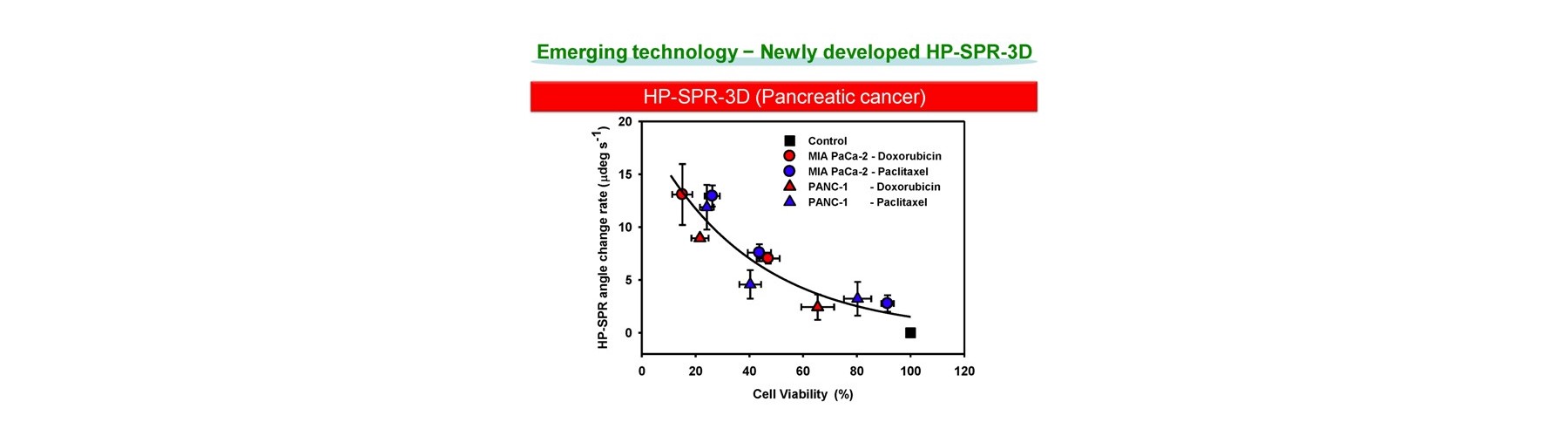
- Early mitochondrial polarization change rate after addition of target compound (s) is revealed as a key to quantitatively predict the final compound efficacy and toxicity against live cells.
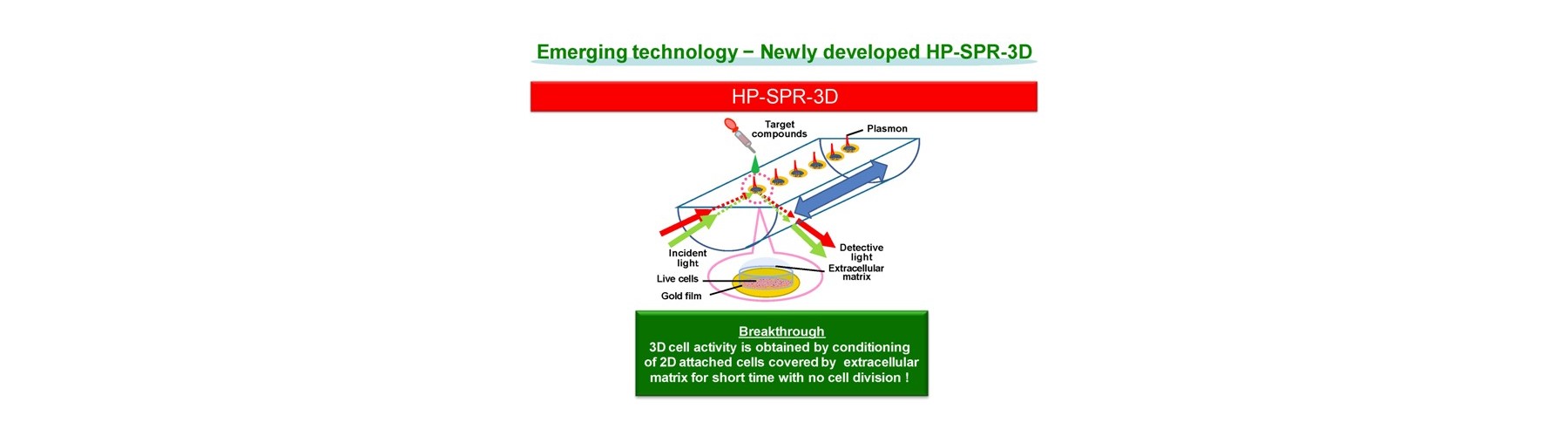
- 3D cell activity is obtained by conditioning 2D attached cells covered by extracellular matrix for short time with no cell division.
Advantages of our technology are as follows.
- Easy handling
- Medium exchange free
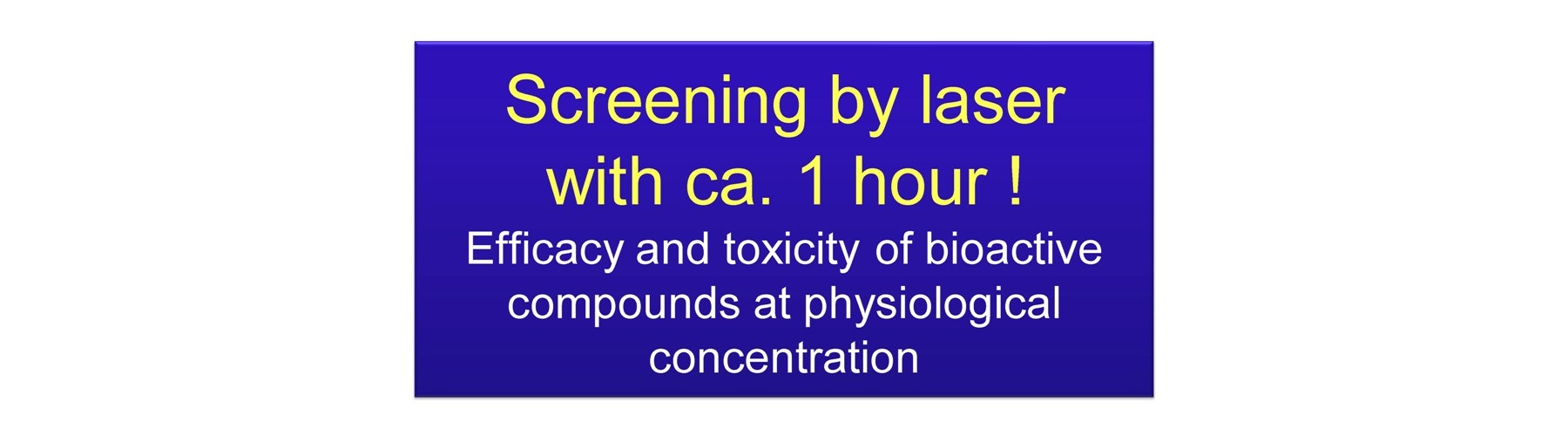
- Rapid decision duration (1h)
- Label free
- Small test error
- Limited cells used (1000)
- Regardless to compound mode of action
- High reliability in polypharmacy
- Physiological concentration evaluation
- Compatibility with conventional results
- Simultaneous evaluation of efficacy and toxicity
Key publications
Kosaihira, A. and Ona, T. Rapid and quantitative method for evaluating the personal therapeutic potential of cancer drugs. Anal Bioanal Chem, 391:1889 (2008).
Ona, T., Nishijima, H., Kosaihira, A. and Shibata, J. Development of cell-based quantitative evaluation method for cell cycle-arrest type cancer drugs for apoptosis by high precision surface plasmon resonance sensor. Biophotonics 9:69910R (2008).
Nishijima, H., Kosaihira, A., Shibata, J. and Ona, T. Development of signaling echo method for cell-based quantitative efficacy evaluation of anti-cancer drugs in apoptosis without drug presence using high-precision surface plasmon resonance sensing. Anal Sci, 26:529 (2010).
Ona, T. and Shibata, J. Advanced dynamic monitoring of cellular status using label-free and non-invasive cell-based sensing technology for the prediction of anti-cancer drug efficacy. Anal Bioanal Chem, 398:2505 (2010).
Ona, T. and Shibata, J. Label-free, rapid and reliable new chemosensitivity test for physiological concentration of anti-cancer drug: evaluation within 1h. J Pharm Sci, 121:132 (2013).
Ona, T. and Shibata, J. Label-free, rapid and reliable new cell-based assay for physiological concentration of anti-cancer and anti-metabolic syndrome compounds: evaluation within 1h. J Pharm Sci, 121:159 (2013).
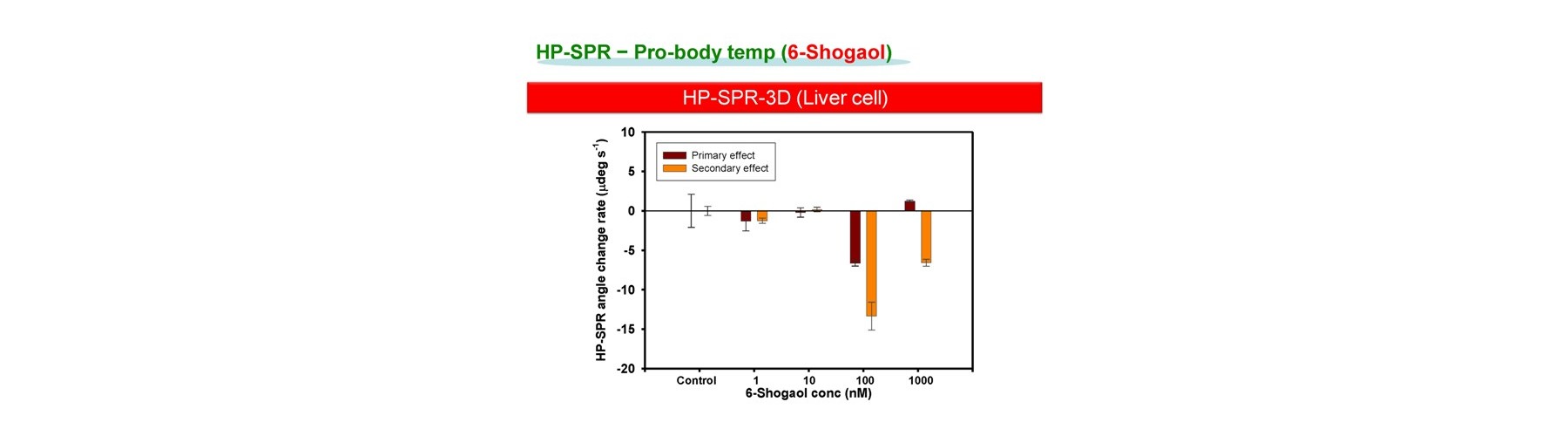
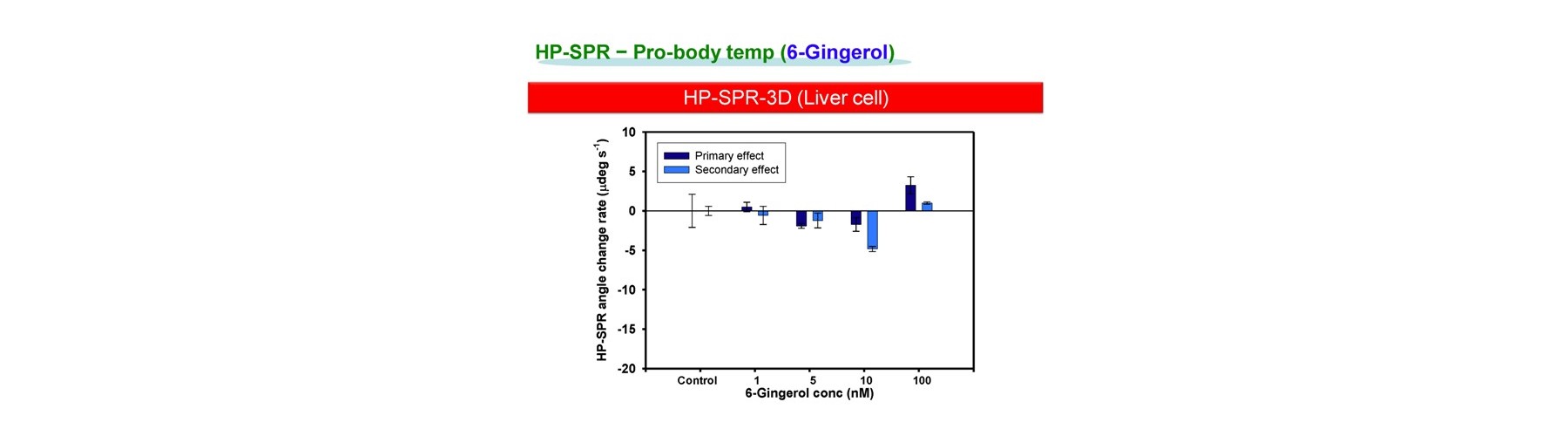
Ona, T. and Shibata, J. Cell-based rapid and quantitative toxicity and efficacy monitoring in consecutive before and after the compound metabolism within liver for two ginger compounds at physiological concentration. ALTEX Proc, 3:24 (2014).
Ona, T. and Shibata, J. Rapid in vivo-like efficacy and toxicity evaluation of target compound using mitochondria activity change within epidermis keratinocyte cell. FRAGRANCE J, 42:50 (2014).
IP rights
Ona, T. and Kosaihira, A. Method of screening substance with anticancerous activity and apparatus therefor. JP2005-17081A (2005); WO 2005/001472 A1 (2005).
Ona, T. and Kosaihira, A. Monitoring of intercellular mitochondrial polarization. WO2007/069692 A1 (2007); CN 200680052043.8 (2008); EP 1 961 824 A1 (2008); US 2011/0003321 A1 (2011); JP2012-93369A (2012).
Ona, T., Method for activating two-dimensional cultured cells similarly to three-dimensional culture or in vivo, and use thereof. WO 2013/039112 A1 (2013).
2. Digestion and absorption model test (for oral administration)
In case of oral administration, chemosensitivity test using target material directly may not provide valid efficacy information because of structural change of the target material by digestion in stomach and others and by adsorption in intestines. To solve this issue, we provide digestion and absorption model test service using digestion enzymes and others to prepare the valid compounds for our chemosensitivity test.